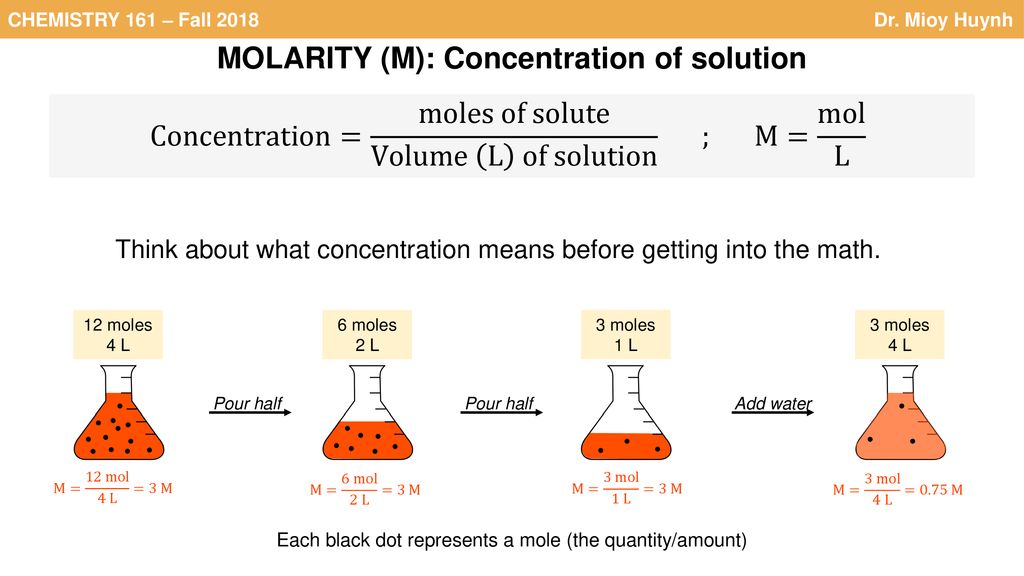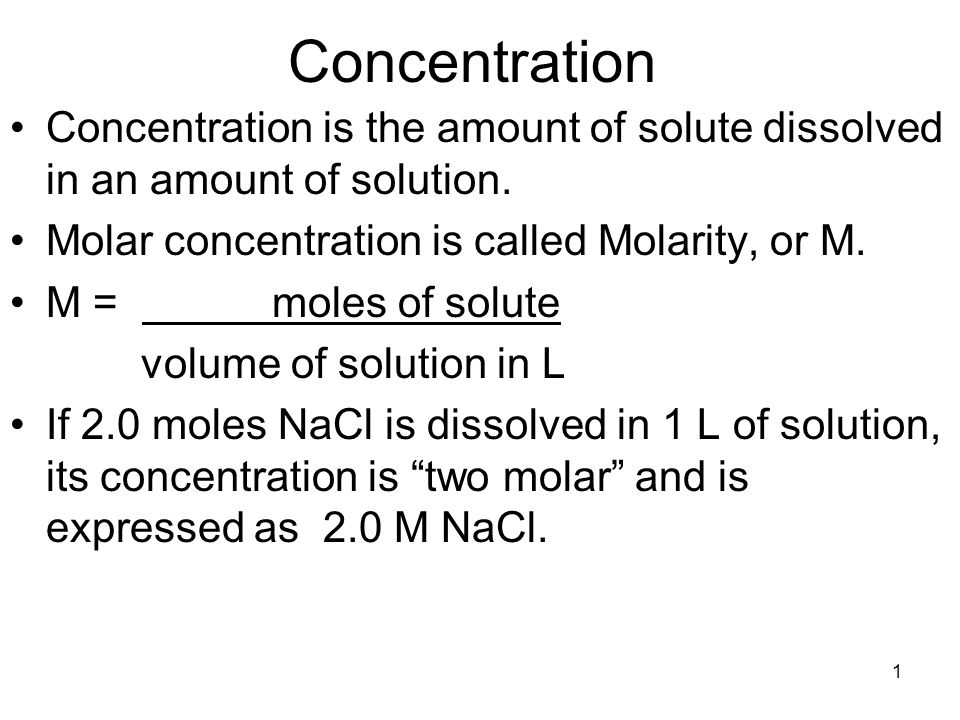
Concentration Concentration is the amount of solute dissolved in an amount of solution. Molar concentration is called Molarity, or M. M = moles. - ppt video online download

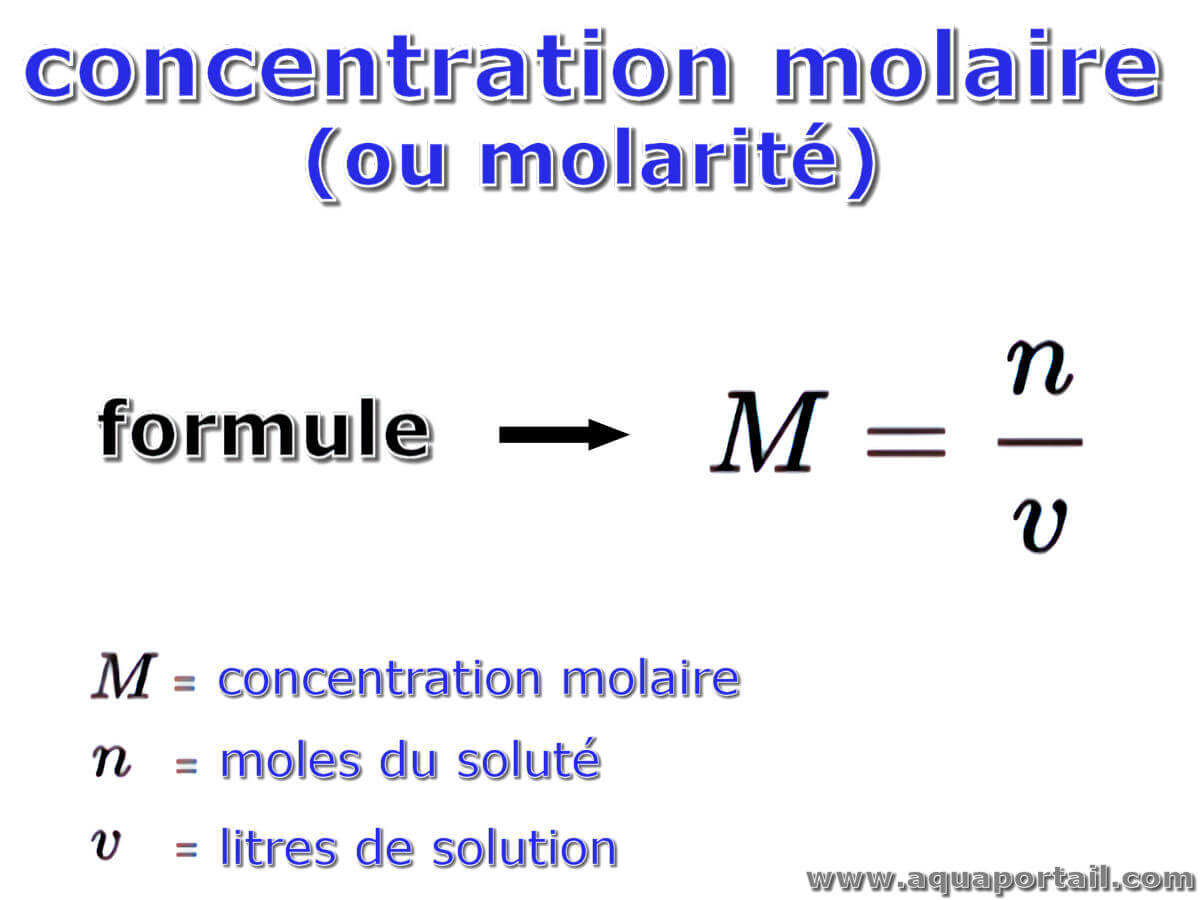
Molarity (Molar concentration) : It is defined as the number of moles of the solute dissolved in per litre of the solution, 1.e xd x 10 c(gm/l) Molarity (M) = Number of

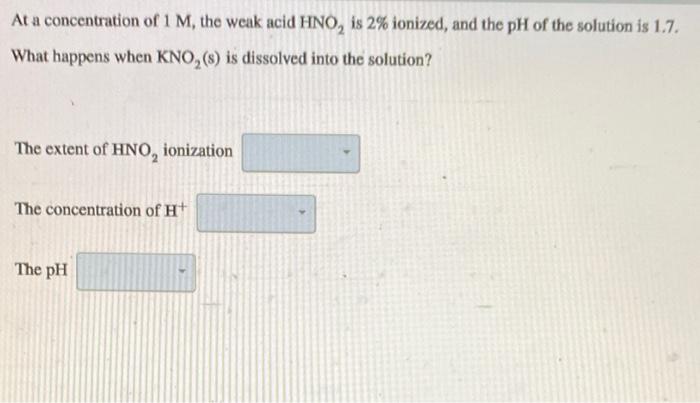
The concentration of acetate ions in 1 M acetic acid (K = 2 x 10-5) solution containing 0.1 M - HCl is (a) 2 x 10-M (b) 2 x 10-'M (c) 2 x 10-M (d) 4.4 x 10-'M

Concentration Concentration is the amount of solute dissolved in an amount of solution. Molar concentration is called Molarity, or M. M = moles. - ppt video online download

Evolution du profil de concentration au cours du temps-J=110 L.h-1 .m-2 | Download Scientific Diagram